ADHESIVES
The strength of adhesive joints or bulk
polymers is proportional to the work G required to propagate a fracture over a
unit area. Thermodynamics provides a
lower bound for G > 2g, where g is the interfacial
free energy. Typical values of G for polystyrene
and plexiglass are several thousand times larger than
this bound. The reason is that weak van der Waals bonds are broken throughout a large volume around
the crack tip. This produces an
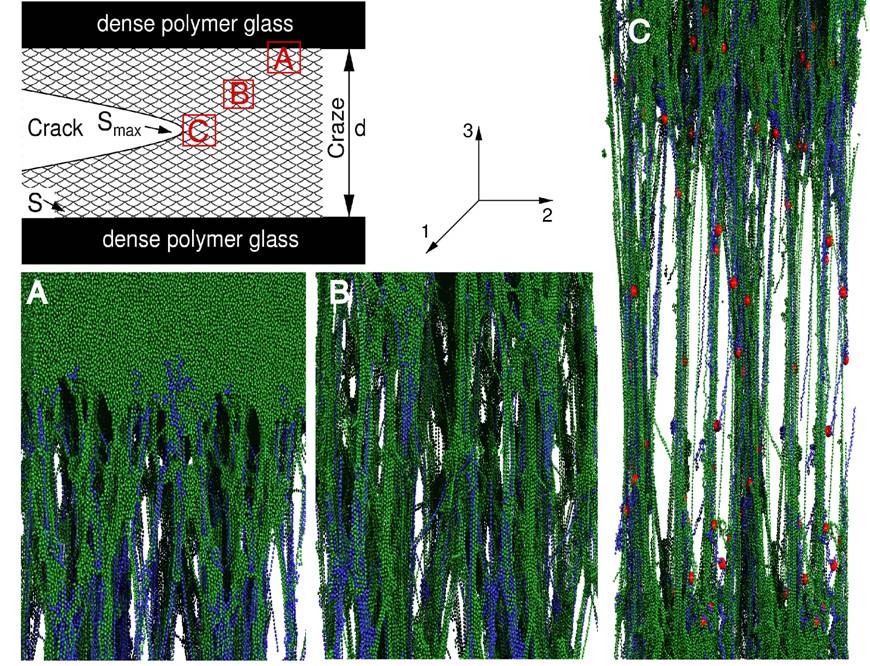
intricate network of fibrils and voids called a craze.
The craze is stronger than the undeformed polymer, so more and more material is deformed
into the craze.
Research in the group has used simulations in
different regions (see below) to calculate the macroscopic fracture energy [102]. Other simulations have analyzed the growth of
crazes in detail to determine how they grow, the stress required to grow them
and how they fail [108]. Work in progress is analyzing the evolution
of the entanglement network during crazing to determine its role.
You will find movies of craze formation here.
Other research has examined what determines
whether adhesives fail at interfaces or in their bulk [82] and found that yield
stresses not free energies determine the location of failure. Work in progress is studying welding of
polymer surfaces that are held together at an elevated temperature to produce
diffusion.